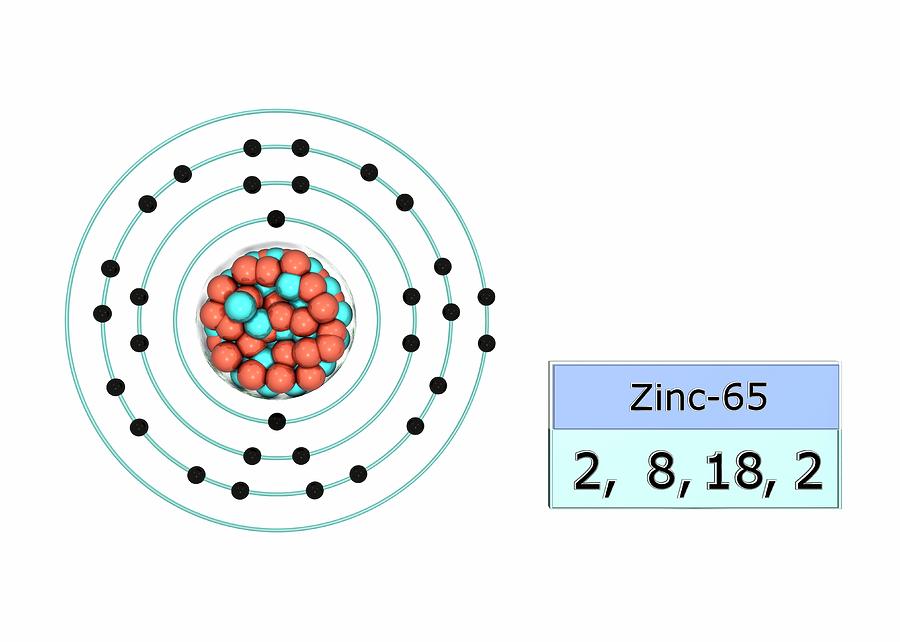
Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table.
Electron configuration zinc. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6 alternatively, write the symbol for the noble gas. (ar)4s^2 3d^10 what is electron configuration for zinc? The electron configurations of the zinc atoms are shown in figure 1.
The electron configuration of a neutral zinc atom is 1s22s22p63s23p63d104s2. Electronic configuration of the zinc atom in. Electron configuration of hydrogen (h) 1s 1:
The electron configuration for zinc is [ar] 4s2 3d10. The last option is the correct one. The zinc atom donates two electrons in the 4s orbital to form a zinc ion (zn 2+ ).
In electronic configuration electrons are arranged in. Located in the iv period. The atomic number of zinc is 30, so 30 electrons are present in its neutral state, so configuration in neutral state will be:
Copper ← zinc → gallium 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. Zinc (zn), atomic # 30, is located in the “d” block in period 4;
It’s electron configuration would be represented as 1s2 2s2 2p6 3s2 3p6 4s2 3d10; The complete electron configuration for zinc is: The electrons are arranged in a semicircle, with the top and bottom of each semicircles being the same size.






