Zn can only form stable cation, zn2+ by releasing two electrons from its outermost shell.
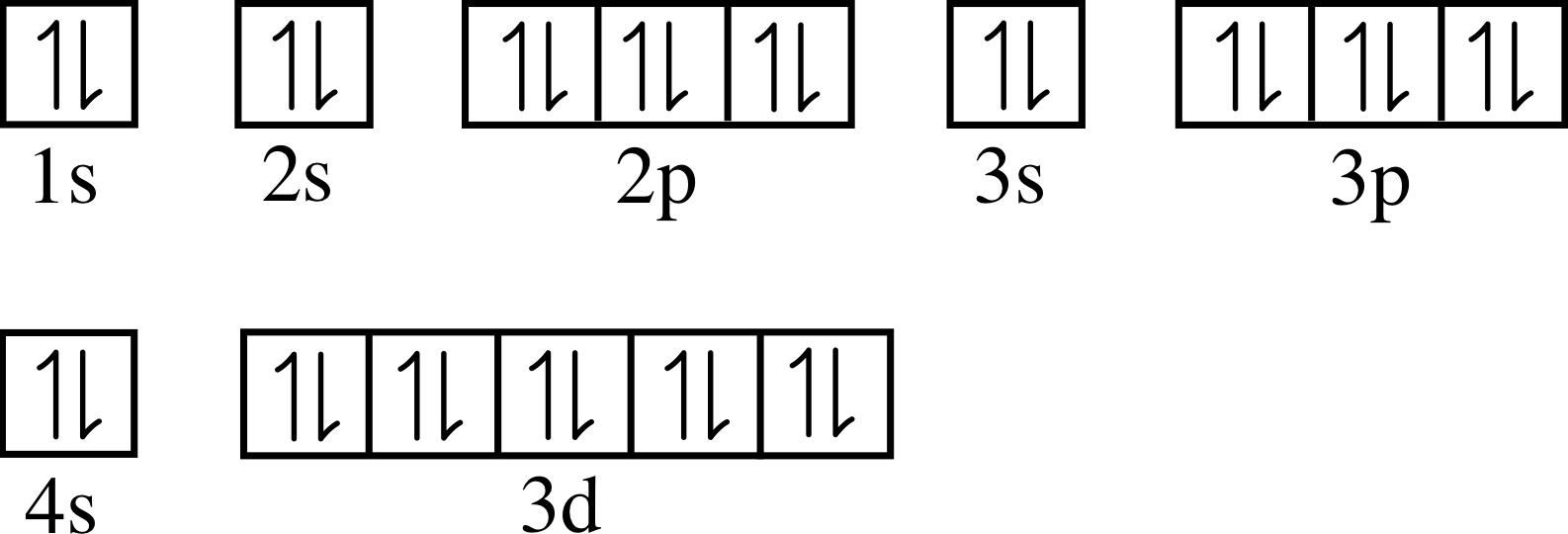
Electron configuration of zinc 2+. The electron configurations of the zinc atoms are shown in figure 1. Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure. 1s2 2s2 2p6 3s2 3p6 3d10 4s2.
1s2 2s2 2p6 3s2 3p6 3d10 4s2. The electronic configuration of the zinc ion is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 Zinc blende, calamine melting point:
Electron configuration of zinc (2, 8, 18, 2) atomic number: Its atomic number is 30, so its complete electron configuration is 1s2 2s22p6. Χ = 1.65 in general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from.
The ground state electron configuration of. Configuration is [ar]4s23d10 the full electron configuration is. How many electrons are in the outermost level of.
5 rows zinc ion (zn 2+) electron configuration. What is the atomic number and electronic configuration of zn? The electrons are arranged in a semicircle, with the top and bottom of each semicircles being the same size.
The chemical symbol for zinc is zn. The atomic number of zinc is 30, which means that all zinc atoms have 30 protons in their nuclei. The electronegativity of zinc is:








