Located in the iv period.
Electron configuration for zinc ion. Zn can only form stable cation, zn2+ by releasing two electrons from its outermost shell. The same rule will apply to transition metals when forming ions. The zinc atom donates two electrons in the 4s orbital to form a zinc ion (zn 2+ ).
Here are energy level curves for both the. 1s2 2s2 2p6 3s2 3p6 3d10 4s2. Zinc atom exhibit +2 oxidation state.
In this case, the zinc atom carries a. The electronic configuration of zinc is 1s2,2s2,2p6,3s2,3p6,4s2,3d10. Electron configuration chart of all elements is mentioned in the table below.the shorthand electron configuration (or noble gas configuration) as well as full.
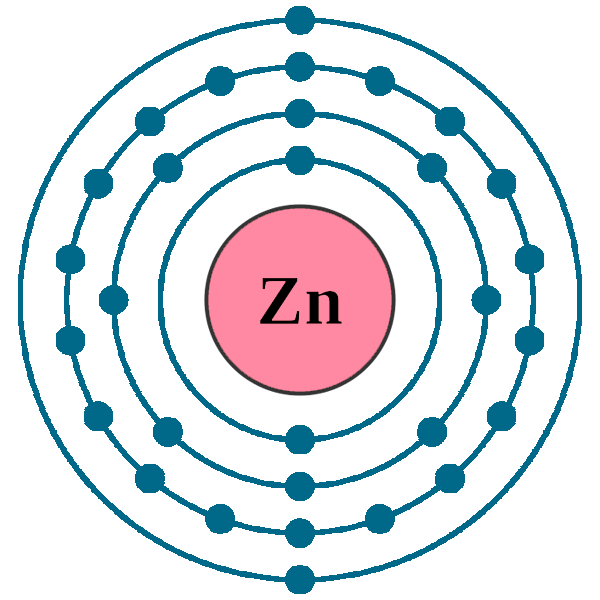
The element zinc (atomic number 30) has the electron configuration. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and. Zinc has only two electrons in its last orbit.
All electrons are paired, and therefore zn (0) is diamagnetic. That is, zirconium is a cation element. The electron configuration shows that.
Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.the chemical symbol for zinc is zn. The zinc atom donates two electrons in the 4s orbital to form a zinc ion(zn 2+). Zn (zinc) is an element with position number 30 in the periodic table.







