Instructor's resource cd to accompany busn, canadian edition [by] kelly, mcgowen, mackenzie, snow;
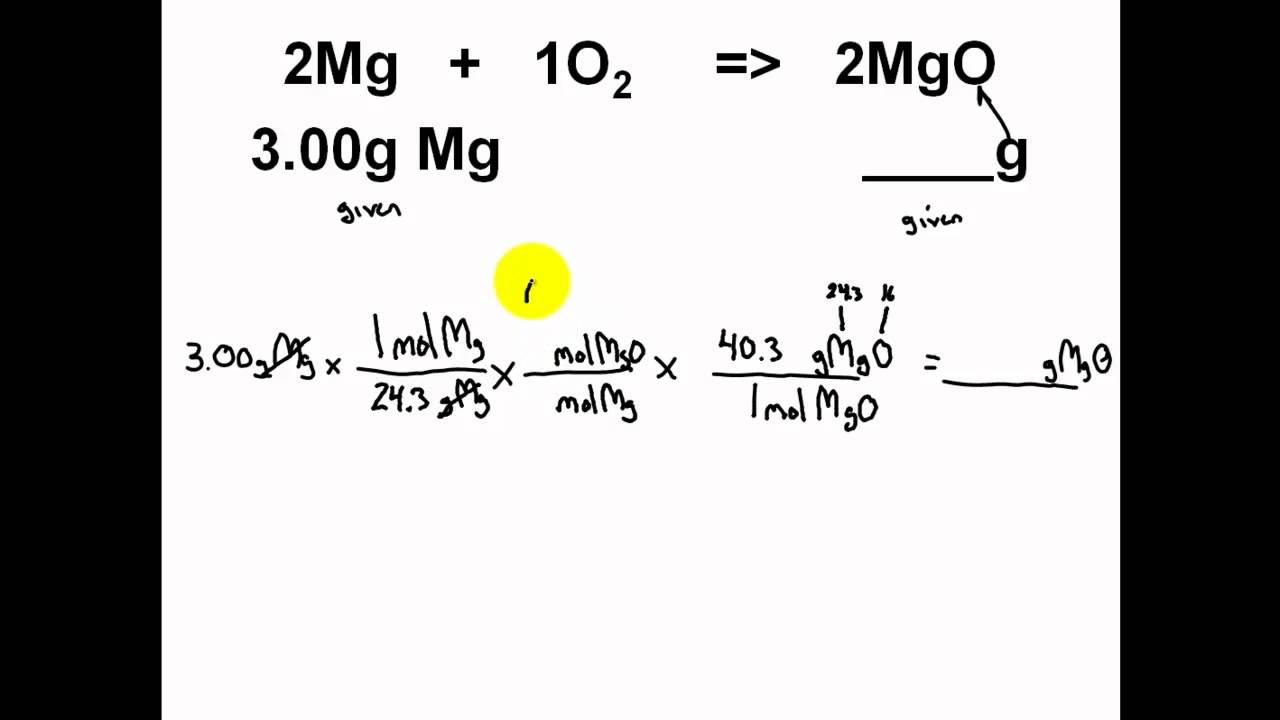
Easy stoichiometry problems. Most students, and many of my (now former) colleagues, find stoichiometry to be one of the most challenging. Science chemistry library chemical reactions and stoichiometry stoichiometry. Simple stoichiometry only (one given, one wanted) limiting reagents only (two given reactants, one wanted product) a mix of both simple stoichiometry and limiting reagent.
Nh 4 no 3 n 2 o + h 2 o e. Gfm of s = 32>br> gfm of h2s = 2×1 + 32 = 34 grams / mole. Name four major categories of stoichiometry problems.
O 3 o 2 d. Silver and nitric acid react. What is the mass of 2 moles of h2s ?
1) write the balanced chemical reaction. A) find the mols of the compound with known mass. Convert the following number of moles of chemical into its corresponding mass in grams.
Questions and answers ( 21,713 ) a 4.00 l sample of hydrogen chloride (hcl) gas. So we have 10.96 moles nh3(g) and 16.44 moles no (g). Solve each of the following problems.
Stoichiometry calculation practice worksheet 1. Empirical formula from mass composition edited. The following stoichiometry road map gives a summary of how to use.









